
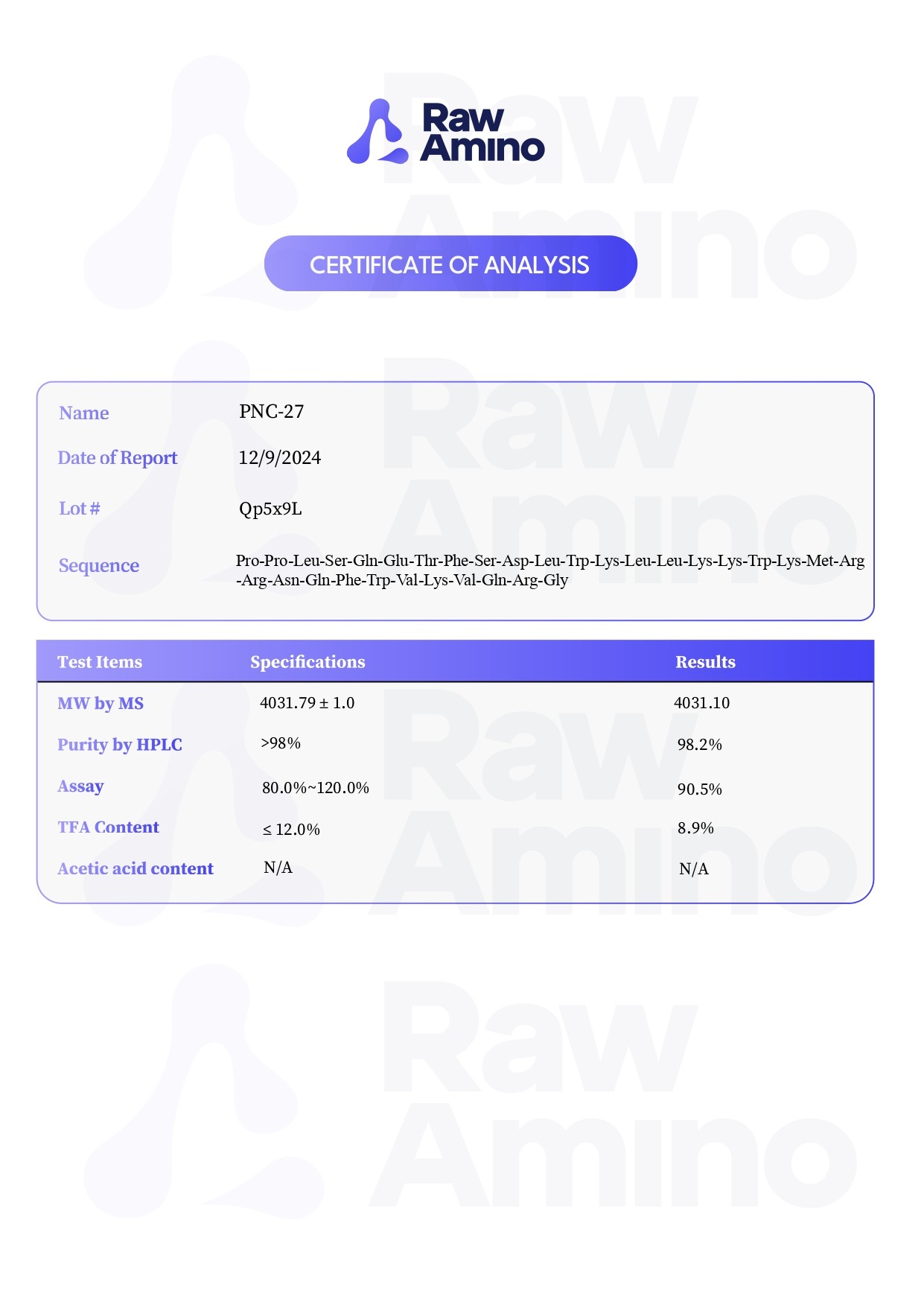
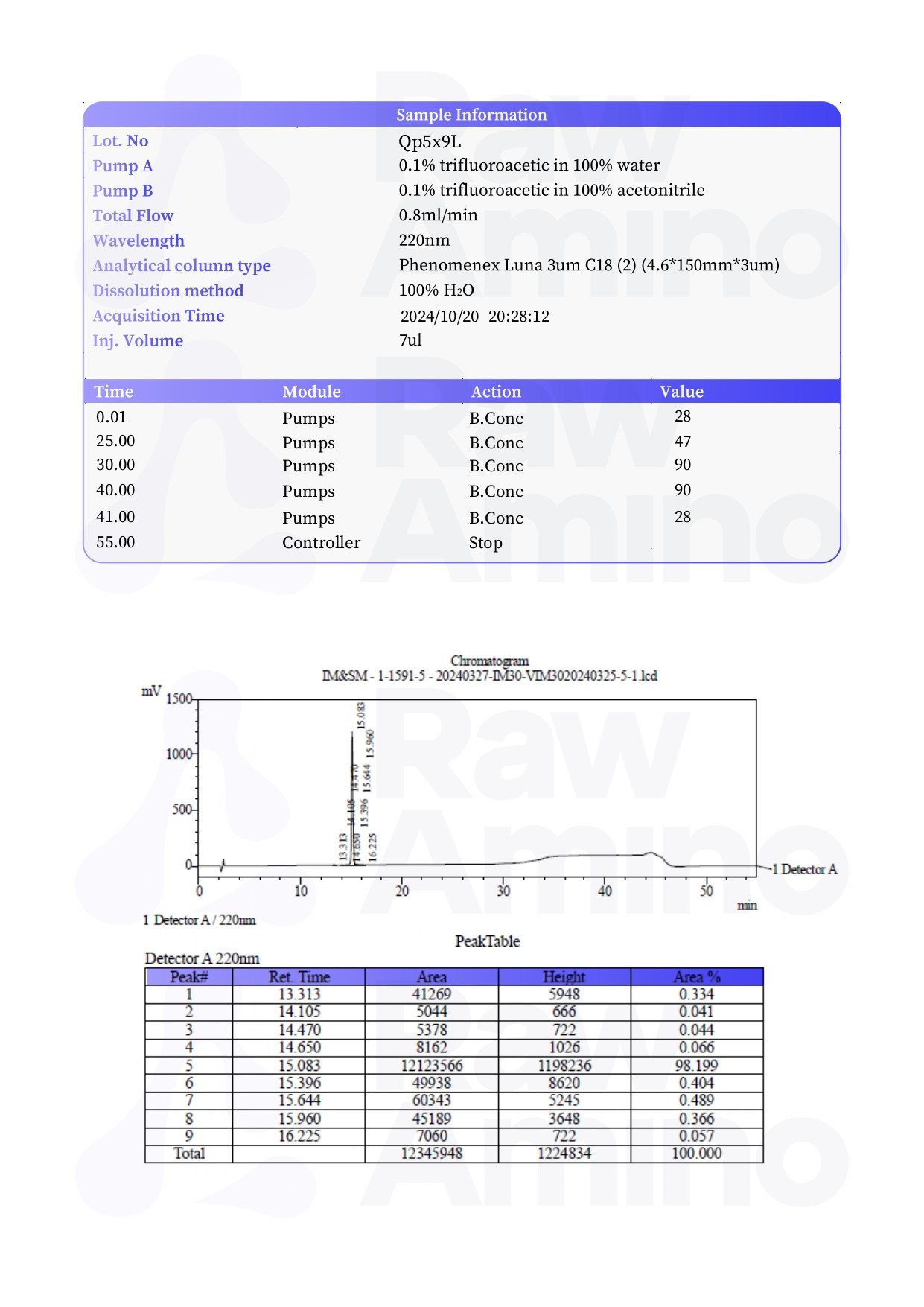
PNC-27 – 5MG
$138.00
Discount per Quantity
| Quantity | Discount | Price |
|---|---|---|
| 5 - 8 | 5% | $131.10 |
| 9 + | 10% | $124.20 |
Scientific Overview of PNC-27
PNC-27 is a synthetic peptide that has been the focus of scientific inquiry for its proposed role in experimental cancer biology. It is part of the broader PNC family of probe proteins and was first described in the early 2000s. Laboratory studies suggest that PNC-27 may interact selectively with malignant cells through an affinity for HDM2, a protein often mislocalized to the plasma membranes of tumor cells. This feature distinguishes cancer cells from their non-malignant counterparts, where HDM2 is typically absent from the membrane surface.
PNC-27 is designed with an HDM2-binding domain derived from the p53 protein, alongside a transmembrane sequence thought to aid membrane association. Research suggests that this structural arrangement may enable the peptide to associate with HDM2 in the plasma membrane, forming ring-like complexes that appear capable of puncturing cell membranes. Observations from experimental models indicate that this may lead to pore formation and disruption of osmotic balance within cancer cells.
Alternative Names: membrane residency peptide (MRP)
PNC-27 Studies and Research Data
Mechanistic Studies on HDM2 Binding
Investigations using molecular modeling, immuno-electron microscopy, and biochemical assays suggest that PNC-27 may form stable complexes with HDM2. These interactions appear to result in oligomeric pore structures on the membranes of transformed cells, while untransformed cells do not display similar alterations. The proposed selective interaction with HDM2 provides a mechanistic rationale for ongoing studies exploring its potential to distinguish between malignant and non-malignant cells.
PNC-27 Research in Leukemia Models
Experimental work has highlighted leukemia cell lines such as U937, HL60, and OCI-AML3 as potential systems where PNC-27 exhibits selectivity. These cell lines are known to express elevated levels of membrane-associated HDM2, which may be central to their sensitivity. In vitro analyses have noted signs consistent with necrosis rather than apoptosis, as evidenced by markers such as lactate dehydrogenase release. The degree of response, however, appears variable across different leukemia models, suggesting possible isoform-dependent factors or other molecular influences.
PNC-27 Explorations in Solid Tumor Cells
In addition to blood-derived models, the peptide has been studied in primary ovarian cancer cells, including mucinous cystadenocarcinoma and papillary serous carcinoma. Reports describe disruption of cellular integrity and release of necrosis-associated markers following exposure, while the overall scope of its influence remains under evaluation. Additional research has also extended to pancreatic, breast, and melanoma models, where the peptide is being examined as part of a broader spectrum of malignancies for its possible role in membrane targeting.
Structural Comparisons with p53 Residues
The sequence of PNC-27 has been compared to critical p53 residues that naturally bind HDM2. Structural overlays suggest that the peptide may replicate features of this interaction while presenting them in a membrane-active context. This mimicry may underpin its proposed ability to anchor into cell membranes while aligning with HDM2, initiating pore assembly. Transfection experiments introducing membrane-localized HDM2 into otherwise non-transformed cells reportedly increase their sensitivity to the peptide, reinforcing the concept of HDM2 as a defining factor in this pathway.
Conclusion
PNC-27 is a synthetic peptide that has attracted scientific attention for its proposed interactions with HDM2 at the plasma membrane of malignant cells. Research suggests that it may promote pore formation and necrosis in select cancer cell models, particularly those with elevated membrane HDM2 expression. Studies across leukemia lines, ovarian cancer cells, and additional solid tumor types continue to explore its mechanistic and structural underpinnings. While results indicate a possible role in selective membrane disruption, variability among models underscores the need for further investigation to fully understand its scope and reproducibility in laboratory systems.
References
- Aguon PM, et al. Experimental PNC-27 Therapy and Massive GI Hemorrhage: A Complication or Coincidence? Am Coll Gastroenterol. 2017.
- Sarafraz-Yazdi E, et al. Anticancer peptide PNC-27 adopts an HDM-2-binding conformation and kills cancer cells by binding to HDM-2 in their membranes. Proc Natl Acad Sci USA. 2010.
- Thadi A, et al. Targeting Membrane HDM-2 by PNC-27 Induces Necrosis in Leukemia Cells But Not in Normal Hematopoietic Cells. Anticancer Res. 2020.
- Davitt K, et al. The anti-cancer peptide, PNC-27, induces tumor cell necrosis of a poorly differentiated non-solid tissue human leukemia cell line. Ann Clin Lab Sci. 2014.
- Sarafraz-Yazdi E, et al. Ex vivo Efficacy of Anti-Cancer Drug PNC-27 in the Treatment of Patient-Derived Epithelial Ovarian Cancer. Ann Clin Lab Sci. 2015.
- Sarafraz-Yazdi E, et al. Abstract# 884: Mechanism of action of PNC-27/-28 anti-cancer peptides. Cancer Res. 2009.
- Sarafraz-Yazdi E, et al. MDM2 protein variants expression in the plasma membrane of cancer cells: A target for anticancer peptide PNC-27. Cancer Res. 2010.
Disclaimer:
The products mentioned are intended solely for laboratory research and in-vitro experimentation. They are not approved for human or animal use of any kind. All details provided are for educational purposes only. By purchasing from this site, you agree to comply with our Terms and Conditions.
Only logged in customers may leave a review.






Reviews
There are no reviews yet.